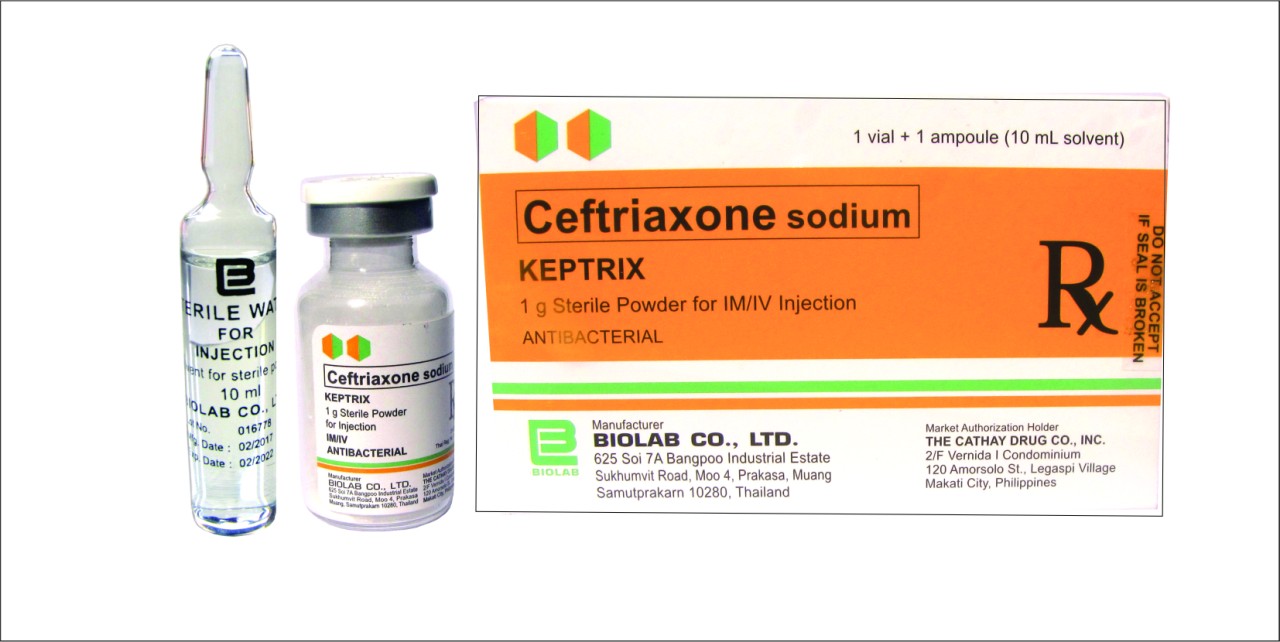
Description
The Cathay Drug Co., Inc.
DESCRIPTION
FORMULATION
Each vial contains:
Ceftriaxone sodium equivalent to Cefuroxime …………………………………1 g
PHARMACOLOGICAL PROPERTIES
Ceftriaxone is a third-generation cephalosporin. It inhibits mucopeptide synthesis in the bacterial cell wall, making it defective and osmotically unstable. Ceftriaxone is highly stable to most beta-lactamases (including penicillinases and cephalosporinases) and therefore, it is active against a wider spectrum of gram-negative bacteria and most Enterobacteriaceae. It is not active against gram-positive cocci as compared to the first and second generation cephalosporins. In vitro, the studies of ceftriaxone and aminoglycosides are synergistic against certain susceptible and resistant strains of P. aeruginosa, S. marcenscens and other Enterobacteriaceae, including Enterobacter cloacae, E. coli, K. pneumonia and P. mirabilis.
PHARMACOKINETICS
Absorption and Distribution
Ceftriaxone demonstrates nonlinear dose-dependent pharmacokinetics due to its protein binding (about 85% to 95% protein bound, depending on the plasma concentration). The plasma half-life of ceftriaxone is not dependent on the dose and varies between 6 and 9 hours; it may be prolonged in neonates. The half-life does not change appreciable in patients with moderate renal impairment, but it may be prolonged in severe renal impairment, especially if there is also hepatic impairment.
Ceftriaxone is widely distributed in body tissues and fluids. It crosses both inflamed and non-inflamed meninges, generally achieving therapeutic concentrations in the CSF. It crosses the placenta and low concentrations have been detected in breast milk. High concentrations are achieved in bile.
Metabolism and Excretion
About 40 to 65% of a dose of ceftriaxone is excreted unchanged in the urine principally by glomerular filtration; the remainder is excreted in the bile and is ultimately found in the feces as unchanged drug and microbiologically inactive compounds.
INDICATIONS
Ceftriaxone sodium (KEPTRIX) is indicated for the treatment of infections caused by pathogens sensitive to Ceftriaxone, namely:
- Lower respiratory tract infections caused by Streptococcus pneumoniae, Staphylococcus aureus, Haemophilus influenzae, Haemophilus parainfluenzae, Klebsiella pneumoniae, Escherichia coli, Enterobacter aerogenes, Proteus mirabilis or Serratia marcescens.
- Skin and skin structure infections caused by Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pyogenes, Viridans group streptococci, Escherichia coli, Enterobacter cloacae, Klebsiella oxytoca, Klebsiella pneumoniae, Proteus mirabilis, Morganella morganii,* Pseudomonas aeruginosa, Serratia marcescens, Acinetobacter calcoaceticus, Bacteroides fragilis* or Peptostreptococcus
- Urinary tract infections (complicated and uncomplicated) caused by Escherichia coli, Proteus mirabilis, Proteus vulgaris, Morganella morganii or Klebsiella pneumoniae.
- Bacterial septicemia caused by Staphylococcus aureus, Streptococcus pneumoniae, Escherichia coli, Haemophilus influenzae or Klebsiella pneumoniae.
- Bone and joint infections caused by Staphylococcus aureus, Streptococcus pneumoniae, Escherichia coli, Proteus mirabilis, Klebsiella pneumoniae or Enterobacter
- Intra-abdominal infections caused by Escherichia coli, Klebsiella pneumoniae, Bacteroides fragilis, Clostridium species (Note: most strains of Clostridium difficile are resistant) or Peptostreptococcus
- Meningitis caused by Haemophilus influenzae, Neisseria meningitidis or Streptococcus pneumoniae.
DOSAGE AND ADMINISTRATION
Intravenous Injection
Ceftriaxone 1 g is dissolved in 10 mL of Sterile Water for Injection. Administered by intravenous injection lasting two to four minutes.
Intravenous Infusion
2 g of ceftriaxone is dissolved in 40 mL, using any of the following calcium-free infusion solutions: 0.9% Sodium Chloride Solution, 2.5% Dextrose+0.45% Sodium Chloride Solution, 5% Dextrose Solution, 10% Dextrose Solution, 5% Levulose Solution, Dextran 6% in Dextrose Solution, Sterile Water for Injection. The infusion should last at least 30 minutes.
Intramuscular Injection
Ceftriaxone 1 g is dissolved in 3.5 mL of 1% Lidocaine solution and administered by deep intramuscular injection. If more than 1 g is to be injected intramuscularly then the dose should be divided between more than one site.
Standard dose
Adults and children over 12 years
Administer 1 to 2 g once daily. In severe cases, the dosage may be raised to a maximum dose of 4 g administered once daily.
Infants and children
20 to 50 mg/kg once daily; for severe infections up to 80 mg/kg once daily may be given.
Neonates
The maximum dose should not exceed 50 mg/kg once daily; intravenous doses in neonates should be given over 60 minutes. Doses above 50 mg/kg should be administered by intravenous infusion only.
Special dosage
In bacterial meningitis in infants and children, treatment begins with doses of 100 mg/kg body weight (not to exceed 4 g) once daily. The duration of therapy is as follows:
|
Microorganism |
Duration of Therapy |
| Neisseria meningitis | 4 days |
| Streptococcus pneumonia | 7 days |
| Haemophilus influenza | 6 days |
| Susceptible Enterobacteriaceae | 10 – 14 days |
Special Populations
Renal impairment
A reduction in dosage may be necessary in patients with severe renal impairment and in those with both impaired renal and hepatic function. Plasma concentrations should be monitored in such patients.
Pregnancy and Lactation
Ceftriaxone should not be used in pregnancy, particularly in the first trimester, unless absolutely indicated. It is excreted in human milk usually in low concentrations. Therefore caution should be exercised if given to lactating women.
CONTRAINDICATIONS
Keptrix is contraindicated in patients with known hypersensitivity to cephalosporin antibiotics.
PRECAUTIONS
As with other cephalosporins, anaphylactic shock cannot be ruled out even if a thorough patient history is taken. Anaphylactic shock requires immediate countermeasures such as intravenous epinephrine followed by a glucocorticoid. In rare cases, shadows suggesting sludge have been detected by sonograms of the gallbladder. This condition is reversible upon the discontinuation of ceftriaxone therapy.
In-vitro studies have shown that ceftriaxone can displace bilirubin from serum albumin. Caution should be exercised when considering ceftriaxone for neonates, especially prematures.
ADVERSE EFFECTS
Adverse effects considered related to ceftriaxone therapy include:
- Gastrointestinal disturbances: loose stools or diarrhea, nausea, vomiting, stomatitis, glossitis
- Hematological changes: eosinophilia, leucopenia, granulocytopenia, hemolytic anemia, thrombocytopenia
- Skin reaction: exanthema, allergic dermatitis, pruritus, urticarial, edema, erythema multiforme
- Other rare adverse effects: headache and dizziness, increased liver enzyme, gallbladder, oliguria, increased serum creatinine, shivering and anaphylactoid reactions
Local adverse effects: in rare cases, phlebitic reaction after IV administration (prevented by slow injection – 2 to 4 minutes).
DRUG INTERACTIONS
No impairment of renal function has been observed after concurrent administration of large doses of Ceftriaxone and loop diuretics (e.g. furosemide). There is no evidence that Ceftriaxone increases renal toxicity of Aminoglycosides. Ceftriaxone has the potential to increase the effects of anticoagulants and to cause a disulfiram-like reaction with alcohol. Unlike many cephalosporins, probenecid does not affect the renal excretion of Ceftriaxone.
Ceftriaxone has the potential to increase the effects of anticoagulants and cause a disulfiram-like reaction with alcohol. Unlike many cephalosporins, probenecid does not affect the renal excretion of ceftriaxone.
OVERDOSAGE – SYMPTOMS, TREATMENT, SUPPORTIVE THERAPY, ANTIDOTE
In appropriately large doses, Ceftriaxone may cause seizures, particularly in patients with renal impairment. If seizure occurs, promptly discontinue the drug. Administer anticonvulsant therapy if clinically indicated.
STORAGE
Store below 25°C. Protect from light.
CAUTION
Foods, Drugs, Devices and Cosmetics Act prohibits dispensing without prescription
AVAILABILITY
1 g per vial


Reviews
There are no reviews yet.