Description
The Cathay Drug Co., Inc.
FORMULATION
Each vial contains:
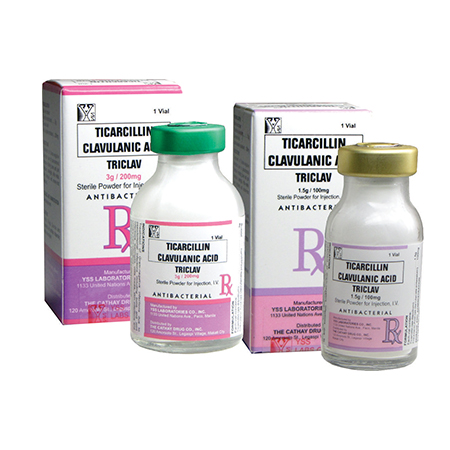
Ticarcillin (as disodium) ………………………………………..……1.5 g
Clavulanic acid (as clavulanate potassium) ……………………. 100 mg
Each vial contains:
Ticarcillin (as disodium) ………………………………………..……3 g
Clavulanic acid (as clavulanate potassium) ……………………. 200 mg
DESCRIPTION
Ticarcillin is a carboxypenicillin which is used in the treatment of severe gram-negative infections particularly those due to Pseudomonas aeruginosa.
Chemical name: (6R)-6[2-carboxy-2-(3-thienyl)-acetamido] penicillinate
Chemical formula: C15H14N2Na2O6S2
Molecular weight: 428.4
Clavulanic acid possess a β-lactam structure resembling that of the penicillin nucleus, except that the fused thiazolidine ring of the penicillins is replaced by an oxazolidine ring. Generally, clavulanic acid has a weak antibacterial activity. It is a potent progressive inhibitor of plasmid-mediated and some chromosomal beta-lactamases produced by gram-negative bacteria including Haemophilus ducreyi, H. influenza, Neisseria gonorrheae, Moraxella catarrhalis (Branhamelle catarrhalis), Bacteroides fragilis, and some Enterbacteriaceae. It is also an inhibitor of the beta-lactamases produced by Staphylococcus aureus. It can permeate bacterial cell walls and can therefore inactivate both extracellular enzymes and those that are bound to the cell.
Chemical formula: C8H8KNO5
Molecular weight: 237.3
PHARMACOLOGICAL PROPERTIES
Mechanism of action
Ticarcillin is bactericidal and is reported to be 2 to 4 times more potent against P. aeruginosa. The activity of ticarcillin against organisms such as Staphylococci, many Enterobacteriaceae, Haemophilus influenzae, and Bacteroides spp. Which are usually resistant due to the production of certain β-lactamases, is enhanced by clavulanic acid whereas activity against P. aeruginosa is not enhanced by clavulanic acid.
Clavulanic acid generally acts as a competitive, and often irreversible β-lactamase inhibitor wherein it consequently enhances the activity of penicillins and cephalosporins against many resistant strains of bacteria. Generally, it is less effective against chromosomally mediated type 1 β-lactamases; that is why many bacterial strains of Cirtrobacter, Enterobacter, Morganella and Serratia spp., and Pseudomonas asruginosa remain resistant with the compound. Klebsiella pneumoniae, and some other Enterobacteriaceae, is also not inhibited by β-lactamase inhibitors. Potassium clavulanate works by blocking the effect of the enzymes thereby rendering the bacteria sensitive to ticarcillin at concentrations readily achieved in the body.
PHARMACOKINETICS
Ticarcillin is not absorbed from the gastrointestinal tract. Peak plasma concentrations ranging from 22 to 35 g/mL are achieved after 0.5 to 1 hour after an intramuscular injection of 1 g. Ticarcillin is 50% bound to plasma proteins while clavulanic acid is approximately 25% bound. A plasma half-life of 70 minutes has been reported. Patients with cystic fibrosis have a shorter half-life which is about 50 minutes in one study, has been attributed to increased renal and non-renal elimination. The half-life is prolonged in patients with renal and hepatic function impairment and in neonates. In patients with severe renal impairment, a half-life of about 15 hours has been reported. Distribution in the body is similar to that of carbenicillin. Relatively high concentrations have been reported in bile, although ticarcillin is principally excreted in the body by glomerular filtration and tubular secretion. It is metabolized to a limited extent and concentrations of 2 – 4 mg/mL are exreted in the urine after intramuscular injection of 1 or 2 g. Up to 90% of a dose is excreted unchanged in the urine, mostly within 6 hours after a dose. Ticarcillin crosses the placenta and only small amounts are distributed into breast milk.
INDICATIONS
Treatment due to β-lactamase producing strains of gram-positive and gram-negative bacteria for the following infections:
- Septicemia, including bacteremia
- Lower respiratory tract infections
- Bone and joint infections
- Skin and skin structure infections
- Urinary tract infections
- Gynecologic infections, including endometritis
- Intra-abdominal infections, including peritonitis
DOSAGE AND ADMINISTRATION
Administer by IV infusion over 30 minutes. Generally continue treatment for at least 2 days after signs and symptoms of infection have disappeared. Usual duration of therapy is 10 to 14 days. For more complicated infections, prolonged therapy may be required.
For adults:
Systemic and urinary tract infections
>60 kg: 3.1 g every 4 to 6 hours for 10 – 14 days
<60 kg: 200 – 300 mg ticarcillin/kg/day in divided doses every 4 to 6 hours for 10 – 14 days
Gynecologic infections:
>60 kg: Moderate infections: 200 mg/kg/day every 6 hours in divided doses
Severe infections: 300 mg/kg/day in divided doses every 4 to 6 hours
For children
>60 kg: Mild to moderate infections 3.1 g every 6 hours
Severe infections: 3.1 g every 4 hours
<60 kg: 200 mg/kg/day (dosed at 50 mg/kg/day) every 6 hours
Severe infections: 300 mg/kg/day (dosed at 50 mg/kg/day) every 4 hours
In renal insufficiency:
Patients on peritoneal dialysis: 3.1g every 12 hours
Patients on hemodialysis: 2 g every 12 hours supplemented with 3.1 g after each dialysis
DIRECTION FOR RECONSITUTION
For 1.5 g/100 mg and 3 g/200 mg vials, dissolve contents in 10 mL and 20 mL of Sterile Water for Injection, respectively.
Use immediately upon reconstitution.
For single use.
STABILITY AND COMPATIBILITY
Administer promptly after reconstitution intravenous injections of Ticarcillin disodium + clavulanate potassium.
Satisfactory antibiotic concentrations of Ticarcillin disodium + clavulanate potassium infusions have been shown to be retained at 25°C in the recommended volumes of the following infusion fluids shown below. Infusions should be used within the time started.
| Intravenous Infusion Fluid | Stability Period at 25°C |
| Sterile Water for Injection | 24 hours |
| Sodium Chloride 0.9% | 24 hours |
| Lactated Ringer’s Solution | 12 hours |
Do not mix Ticarcillin disodium + clavulanate potassium infusions with blood products, intravenous lipid emulsions and other proteinaceous fluids. It is not sufficiently stable in bicarbonate infusion fluids.
CONTRAINDICATIONS
History of hypersensitivity to β-lactam antibiotics.
PRECAUTIONS
Give with caution in patients with renal impairment. Renal, hepatic, hematologic function and electrolyte concentrations should be monitored periodically upon prolonged therapy. Avoid contact since skin sensitization may occur. Therapy should be discontinued upon occurrence of bleeding manifestations.
Drug Interactions
Probenecid decreases renal tubular secretion of ticarcillin. Concurrent administration of probebnecid delays the renal excretion of ticarcillin but not with clavulanic acid.
Pregnancy and Lactation
Cannot be recommended for use in pregnancy since there is no experience yet of its use; use during pregnancy when the potential benefits outweigh the potential risks associated with the treatment; may be used during lactation period.
ADVERSE EFFECTS
Cholestatic jaundice and hepatitis; nausea, vomiting, coagulation disorders, haemorrhagic cystitis (more frequent in children), injection site reactions, Steven Johnson syndrome, toxic epidermal necrolysis, hypokalemia, eosinophilia.
OVERDOSAGE
Symptoms: neuromuscular hyperexcitability, seizures. The drug may be removed from the circulation via haemodialysis.
STORAGE
Store at temperatures not exceeding 2 – 8°C. Do not freeze.
AVAILABILITY
Ticarcillin disodium + clavulanate potassium (Triclav) 1.5 g/100 mg: 10 mL type III clear glass vial in box of 1’s.
Ticarcillin disodium + clavulanate potassium (Triclav) 3 g/200 mg: 20 mL type III clear glass vial in box of 1’s.
CAUTION
Foods, Drugs, Devices and Cosmetics Act prohibits dispensing without prescription.
Manufactured by
YSS LABORATORIES CO., INC.
1133 U.N. Ave., Paco, Manila
Distributed by
THE CATHAY DRUG CO., INC.
2/F Vernida 1 Condominium
120 Amosolo St., Legaspi Village, Makati City

Reviews
There are no reviews yet.