Description
The Cathay Drug Co., Inc.
FORMULATION
Each dry powder for inhalation capsule contains:
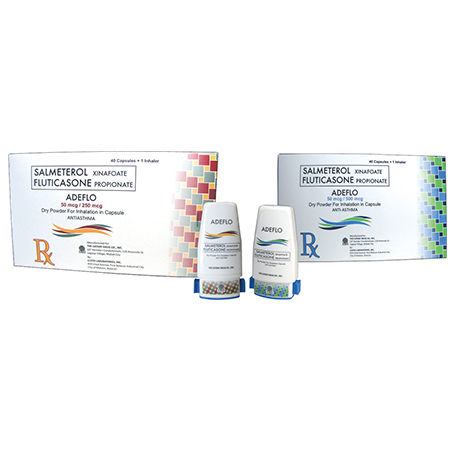
Adeflo 50 mcg/250 mcg
Salmeterol (as xinafoate)…………………. 50 mcg
Fluticasone (as propionate) …………….. 250 mcg
Adeflo 50 mcg/500 mcg
Salmeterol (as xinafoate)…………………. 50 mcg
Fluticasone (as propionate) …………….. 500 mcg
Clinical Pharmacology
The above combination represents 2 classes of medications (a synthetic corticosteroid and a selective, long acting beta-adrenergic receptor agonist) that have different effects on clinical and physiological indices.
Fluticasone Propionate
It is a synthetic trifluorinated corticosteroid with potent anti-inflammatory activity. In-vitro assays using human lung cytosol have established fluticasone propionate as a human glucocorticosteroid receptor agonist with an affinity 18 times greater than dexamethasone, almost twice that of beclamethasone – 17-monopropionate (BMP), the active metabolite of beclomethasone dipropionate and over 3 times that of budesonide. Inflammation is an important component in the pathogenesis of asthma. Corticosteroids have been shown to inhibit multiple cell types (e.g. mast cells, eosinophils, leukotrienes and cytokines) involved in the asthmatic response. These anti-inflammatory actions of corticosteroids contribute to their efficacy in asthma. Inflammation is also a component in the pathogenesis of chronic obstructive pulmonary disease (COPD).
Salmeterol Xinafoate
It is a long acting beta2-adrenoceptor agonist (LABA). The pharmacologic effect of salmeterol xinafoate is part attributable to stimulation of intracellular adenyl cyclase, the enzyme that catalyzes the conversion of adenosine triphosphate (ATP) to cyclic-3’.5’-adenosine monophosphate (cyclic AMP). Increased cyclic AMP levels caused relaxation of bronchial smooth muscle and inhibition of release mediators of immediate hypersensitivity from cells, especially from mast cells. In vitro tests show that salmeterol is a potent and long-lasting inhibitor of the release of mast cell mediator, such as histamine, leukotrienes and prostaglandin D2, from human lung. Salmeterol inhibits histamine-induced plasma protein extravasation and inhibits platelet-activating factor-induced eosinophil accumulation in the lungs of guinea pigs when administered by the inhaled route.
INDICATIONS
For the maintenance and treatment of mild, moderate and severe persistent asthma in patients more than 5 years of age and in patients with chronic obstructive pulmonary disease
DOSAGE AND ADMINISTRATION
Adults and children 12 years of age and older
1 inhalation of Salmeterol xinafoate 50 mcg + fluticasone propionate 250 mcg or 50 mcg/500 mcg twice a day (morning and evening approximately 12 hours apart) or as prescribed by the physician.
The recommended initial dosage for patients aged 12 years and older is based on asthma severity.
Improvement in asthma control following inhaled administration of ADEFLO (salmeterol + Fluticasone propionate) can occur within 30 minutes of beginning treatment, although maximum benefit may not be achieved for 1 week or longer after starting treatment. Individuals may experience a variable time to onset and degree of symptom relief.
For patients who do not respond adequately to the starting dosage after 2 weeks of therapy, replacing the current strength of ADEFLO (Salmeterol+Fluticasone propionate) with a higher strength, adding additional inhaled corticosteroid, initiating oral corticosteroids) should be considered.
Chronic Obstructive Pulmonary Disease
The recommended dosage for patients with COPD is 1 inhalation of Adeflo 50 mcg/250 mcg or 50 mcg/500mcg twice daily (morning and evening approximately 12 hours apart)
If shortness of breath occurs in between doses, an inhaled short acting beta-2 agonist should be taken for immediate relief.
Instruction for Use/Handling
- Remove the cap
- Steadily hold the base and open the inhaler turning the nozzle counterclockwise
- Introduce the capsule into the loading chamber
- Close the nozzle turning it counterclockwise
- Thoroughly press the two buttons, keeping the inhaler in vertical position
- Deeply breathe out
- Introduce the whole nozzle into the mouth and breath in, closing the lips (avoid air passing around the nozzle). Try it again three to four times till the capsule is completely empty.
- After use, extract the empty capsule and close the inhaler.
CONTRAINDICATIONS
Fluticasone Propionate
- Known hypersensitivity or allergy to any ingredient.
Salmeterol Xinafoate
- Hypersensitivity to salmeterol or any component
- Previous history of paradoxical bronchospasm
ADVERSE EFFECTS
Salmeterol may cause the fine tremor of skeletal muscle (particularly the hands), palpitations, tachycardia, nervous tensions, headaches, peripheral vasodilation, and rarely muscle cramps. Inhalation causes fewer side effects than systemic administration and the more selective beta-2-agonist cause fewer side effect than less selective beta agonist. Potentially serious hypokalemia has been reported after large doses. Hypersensitivity reactions have occurred, including paradoximal bronchospasm, angioedema, urticarial, hypotension and collapse.
Fluticasone hypersensitivity reactions may occur. Eosinophilic conditions including Churg-Strauss Syndrome, have been reported rarely, in most cases following a transfer from oral corticosteroid therapy. The adverse effects of corticosteroid may result from unwanted mineralocorticoid or glucocorticoid actions, or from inhibiton of the hypothalamic-pituitary-adrenal axis. Despite the fact that inhaled fluticasone is generally thought to lack systemic effects at therapeutic doses, a study in 25 healthy subjects indicated that fluticasone propionate as single inhaled doses of 250, 500 and 100 ug did produce a reduction in plasma cortisol, indicating suppression with fluticasone, particularly at high doses, and the effect may be more marked with repeated than with single doses.
PRECAUTIONS
Fluticasone Propionate
- Infection of nasal passages and sinuses
- Recent nasal surgery or injury
- Pregnant and lactating mothers
- May affect growth in children
- Not recommended for children below 4 years old
Salmeterol Xinafoate
- Avoid use as sole agent for asthma control
- Patients should be instructed to stop salmeterol when they have breakthrough asthma symptoms or acute asthma exacerbation; shift to short active selective beta-agonist
- Avoid use in pregnancy
DRUG INTERACTIONS
Salmeterol and other beta-2 agonist with corticosteroids, diuretics or xanthines increases the risk of hypokalemia, and monitoring of potassium concentrations is recommended in severe asthma, where such combination therapy is the rule. For an outline of interactions associated with sympathomimetics in general.
Concurrent use of fluticasone with barbiturates, carbamazepine, phenytoin, primidone or rifampicin may enhance the metabolism and reduce the effects of systemic corticosteroids. Conversely oral contraceptives or ritonavir may increase plasma concentrations of corticosteroids. Use of corticosteroids with potassium depleting diuretics, such as thiazides or furosemide may cause excessive potassium loss.
WARNING
In case of powder residues, blow through the nozzle or use a brush and periodically wash with water.
STORAGE CONDITION
Store at temperatures not exceeding 30°C.
AVAILABILITY
Alu-Alu Blister Pack x 8’s (Box of 40’s)
Alu-Alu Blister Pack x 8’s (Box of 32’s)
Manufactured by:
Lloyd Laboratories, Inc.
#10 Lloyd Ave., First Bulacan Industrial City
City of Malolos, Bulacan
Manufactured for:
The Cathay Drug Co., Inc.
2/F Vernida I Condominium, 120 Amorsolo St., Legaspi Village Makati City
Date of revision: July 2, 2019


Reviews
There are no reviews yet.